Editor’s Note: Alexis North is a third-year graduate student in Conservation of Archaeological and Ethnographic Materials at UCLA. She specializes in the conservation of archaeological objects and is working at the Michael C. Carlos Museum at Emory University this summer, preparing a group of objects for display here at HMNS. Read the first blog from her series here.
You may think of metal as a strong, impervious material. It’s used in bridge and building construction, and many of the tools we use today are made of metal (like silverware, hammers and screwdrivers, medical scalpels, etc.). Despite its strength, however, metal can be one of the more fragile materials found in archaeological sites. This is because different types of metal can very easily corrode in the presence of moisture and salts, both of which are found in the burial soils of archaeological sites. If you’ve ever seen red rust on an iron fence, or an old penny turn green, then you’ve seen what corrosion can look like.
Five of the objects I am working on this summer are made of copper alloy. An alloy is a mixture of metals. Copper is most often alloyed with silver, tin, arsenic or lead (or any combination of those) and the resulting mixture will have different strengths and working properties depending on the components and the proportions of those components. Here at the conservation laboratory at the Carlos Museum, one way we can determine which metals are present in an alloy is by using X-ray fluorescence spectroscopy (XRF).
XRF analysis uses X-rays to excite the electrons within a material. These electrons jump to a higher energy level when they come into contact with the X-rays. The electrons of each element give off a characteristic amount of energy when they return to their unexcited state.
By measuring the amounts of energy emitted, we can determine which elements comprise a certain object. Here, the XRF spectrum of the cat figurine seen in my first blog post shows that the metal is an alloy of copper (Cu) and lead (Pb), with a possible trace amount of silver (Ag). The iron (Fe) most likely comes from the burial environment.
 XRF spectrum of 1999.001.043, revealing copper and lead as major components.
XRF spectrum of 1999.001.043, revealing copper and lead as major components.
Copper and its alloys are susceptible to several different types of corrosion, some of which are good or protective corrosion, and some of which can be very damaging to the objects. After a copper alloy object is buried, it forms a protective layer of copper oxide (cuprite) on its surface. Cuprite can be bright to deep red in color, and will preserve the original surface of the object, even when additional corrosion layers form on top. That upper layer of corrosion is usually made of copper carbonates, called malachite and azurite. These compounds are bright green and blue in color, respectively, and have historically been used as pigments, in Egypt and elsewhere.
The real bad boys of copper corrosion are the copper chlorides. These appear as a pale turquoise green compound, usually in spots on the metal’s surface. When copper metal comes into contact with chloride anions, it forms deep pits full of copper chlorides. These pits disrupt the metal’s surface, damaging the original appearance of the object and obscuring surface details. These pits are also autocatalytic, meaning that once one appears, it will continue to grow and form additional pits until the copper chlorides are removed. This cycle of corrosion is commonly called “Bronze Disease,” like a kind of copper Chicken Pox!
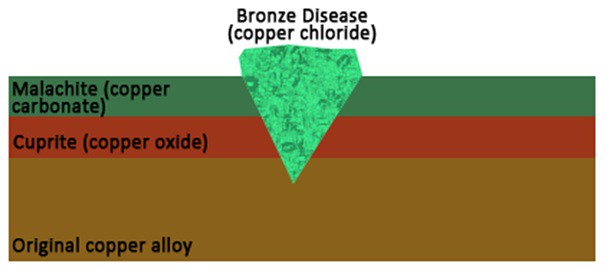 Schematic diagram of copper alloy object with various types of corrosion products.
Schematic diagram of copper alloy object with various types of corrosion products.
All five copper alloy objects that I am working on show evidence of Bronze Disease, as well as malachite and cuprite formations. The cat figurine has very little corrosion, and will not require much treatment at all before it will be ready to pack up and ship to the HMNS. This mirror, on the other hand, has significant corrosion all over its surface. In the detail image on the right, you can see where I’ve found an area of Bronze Disease, and the powdery light green copper chlorides are erupting onto the surface.
 Before treatment image of copper alloy mirror (left) and close-up image of Bronze Disease pit with copper chloride corrosion products (right).
Before treatment image of copper alloy mirror (left) and close-up image of Bronze Disease pit with copper chloride corrosion products (right).
Treating Bronze Disease is a two-step process. First, the copper chlorides must be mechanically removed. I do this using a variety of tools, including scalpels and dental tools (if they work for cleaning your teeth, then they should work for cleaning copper!). The copper chlorides are gently scraped away, while making sure that I don’t damage the rest of the mirror’s surface. The pits made by the copper chlorides are carefully cleaned out, so they can then be chemically treated to help prevent the formation of new copper chlorides. Once the corrosion products have been removed, the objects are treated with Benzotriazole (BTA), a corrosion inhibitor that forms a stable coating with the superficial copper ions, so they cannot react with any chloride ions which may come around.
Corrosion cannot be stopped completely, but these treatments help to significantly slow down the deterioration process, allowing the objects to continue to be displayed and studied. While the corrosion may not be vanquished entirely, with careful consideration the right conservation treatment can be undertaken, allowing these objects to be enjoyed both by scholars and museum visitors like you for many years to come!
References:
“Benzotriazole,” Conservation and Art Material Encyclopedia Online (CAMEO), Museum of Fine Arts, Boston, http://cameo.mfa.org/wiki/Benzotriazole, accessed 7/16/2013
Scott, David A. Copper and Bronze in Art: Corrosion, Colorants, and Conservation. Los Angeles: Getty Publications, 2002.







