Our Posts
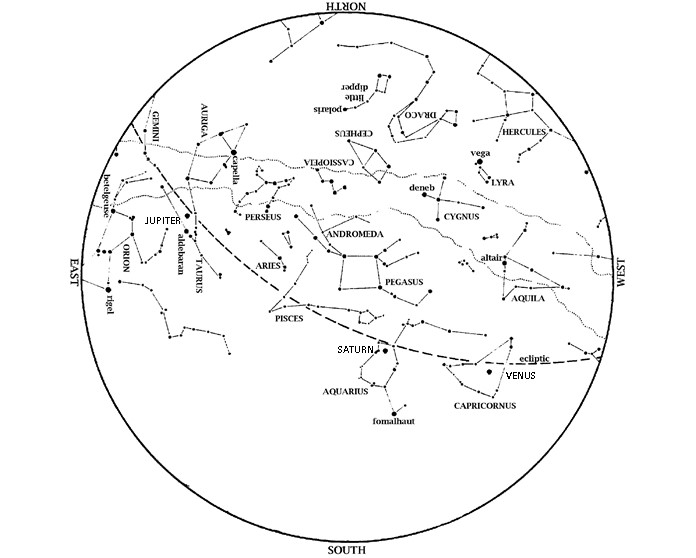
Editor’s Note: Look to the sky as HMNS Astronomer talks winter solstice for the final sky happenings of 2024. Thank you for watching the stars alongside us. Saturn is an evening object. Look in the south at dusk. Venus is also in the evening sky. Look low in the southwest at dusk, near the […]
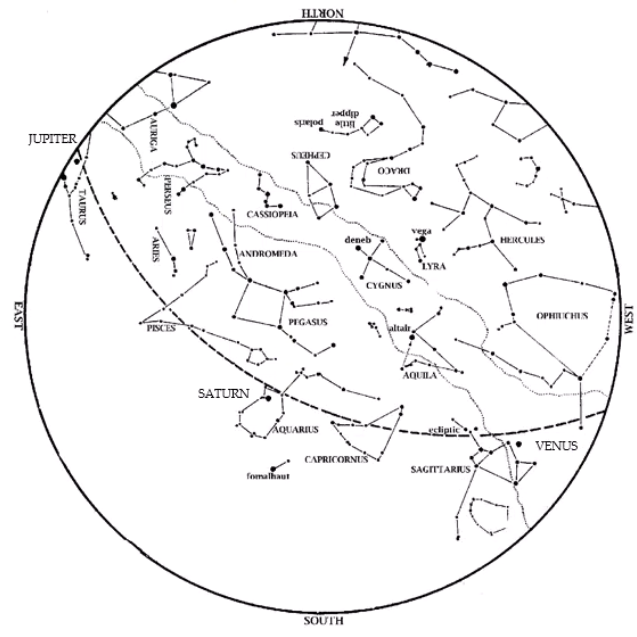
Editor’s Note: HMNS Astronomer James Wooten reminds us to adjust our clocks as Daylight Savings Time ends this month. Saturn is an evening object. Look in the southeast at dusk on Nov. 1, closer to the south by Thanksgiving. Venus is also in the evening sky. Look low in the west-southwest at dusk, near […]
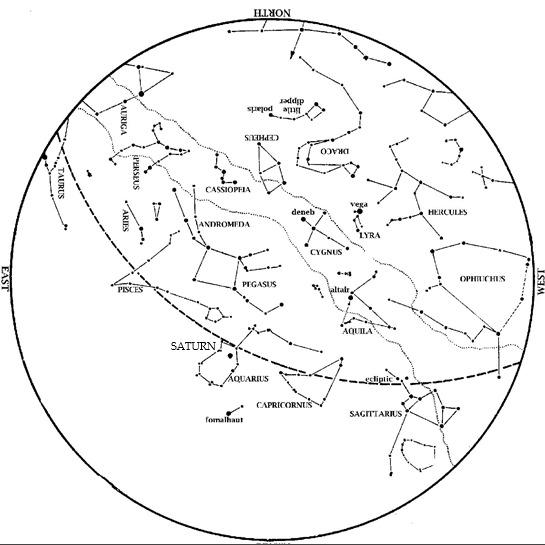
Editor’s Note: Comet Tsuchinshan-ATLAS can be spotted with the naked-eye this month. HMNS Astronomer James Wooten explains. Saturn is now an evening object. Look in the southeast at dusk. Venus has also emerged into the evening sky. Look low in the west-southwest at dusk, near the point of sunset. Jupiter is high, almost overhead at […]

At 7:44 am on Sunday, September 22, 2024, the Sun is overhead at the equator, marking the autumnal (fall) equinox. Also, October 2024 marks the 35th anniversary of our Cockrell Sundial. Here at the Museum, we’ll mark the occasion with a special event on Saturday, September 21, brought to you by Rice University along with […]
Look for a small ‘bite’ taken out of the Moon.
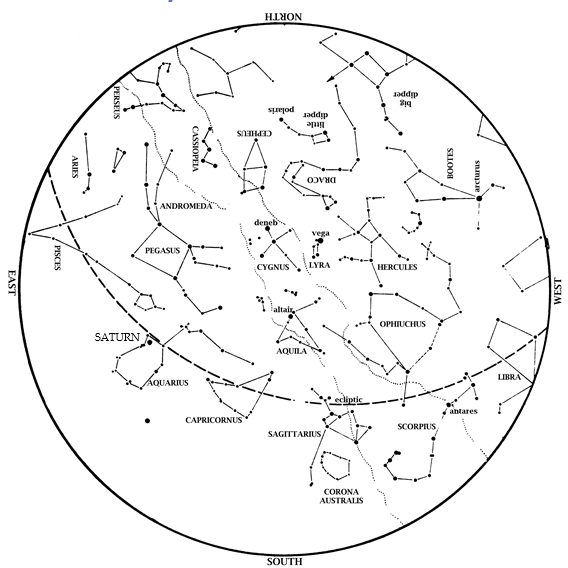
Editor’s Note: The month of August brings the Perseid meteor shower. HMNS Astronomer James Wooten explains what to expect with upcoming sky happenings. Saturn is now a late evening object! It rises in the east-southeast by 10:11 pm on August 1 and by 8:15 (during twilight) on August 31. Saturn is up literally all night […]
Editor’s Note: The July summer heat is not cooling off any time soon and HMNS Astronomer James Wooten is here to explain why that is. Saturn is in the south-southwest at dawn. Mars is also in the morning sky, in the east at dawn. Mars brightens each morning this month as it approaches Jupiter. […]
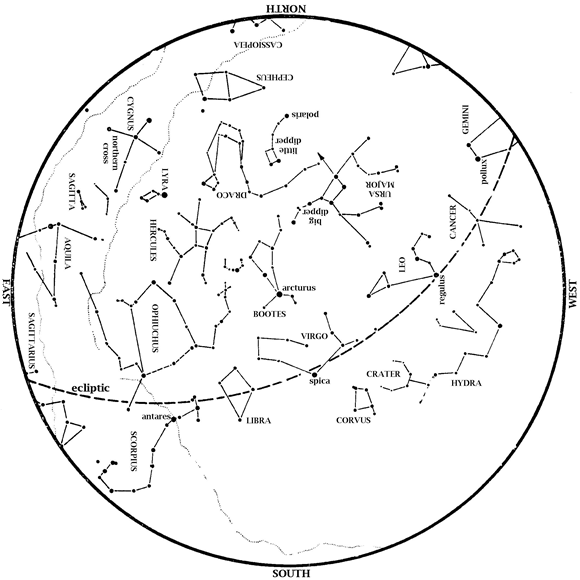
Editor’s Note: Summer Solstice is upon us this month and HMNS Astronomer James Wooten points to the Sun’s highest point in our sky. Saturn is in the southeast at dawn. Mars is also in the morning sky, in the east at dawn. Mars and Saturn easily outshine the very dim stars around them. Venus […]

Editor’s Note: Summer is on the horizon and HMNS Astronomer James Wooten is explaining the signs in the stars above Houston’s skyline. Mars and Saturn are low to the east-southeast horizon at dawn. Having overtaken Saturn, Mars pulls away from it each morning in May. During May, Mars and Saturn appear slightly father from the […]

Editor’s Note: The Total Solar Eclipse is emerging over Texas and HMNS Astronomer James Wooten is giving us the details down to the minute. Jupiter is low in the west at nightfall. No star at night is as bright. Watch as Jupiter appears slightly lower in the sky each April evening until it sets in […]
Editor's Picks The Real Moon Hoax That You Haven’t Heard Of Is Darwin relevant today? Oh The Hermannity! The Story of Houston’s Most Beautiful Green Space A Few Member Benefits Most HMNS Members Don’t Know About What The Loss Of The Museu Nacional in Rio de Janeiro’s Collections Means To The World What Is The Deal With Brontosaurus?!
5555 Hermann Park Dr.
Houston,Texas 77030
(713) 639-4629
Get Directions Offering varies by location Donate Today
13016 University Blvd.
Sugar Land, Texas 77479
(281) 313-2277
Get Directions Offering varies by location Donate Today
21901 FM 762 Rd.
Needville, Texas 77461
(979) 553-3400
Get Directions Offering varies by location Donate Today

